All products
Guide
This text is machine translated.
Batteries, the clever power dispenser with a very long tradition
It has now been over 200 years since her work was proven to have been the first working battery with galvanic cells, with several zinc and copper plates. Although the Volta`sche pillar, as it was called at the time, was still large and awkward to handle, it paved the way to electrical engineering and many other interesting discoveries. And to this day, the operating principle of a battery has not changed much.
What is a battery?
Battery or rechargeable battery, what do I use when?
How is a battery structured?
How does a battery work?
Battery types compared
Battery sizes in comparison
Special battery comparison table
What is a battery?
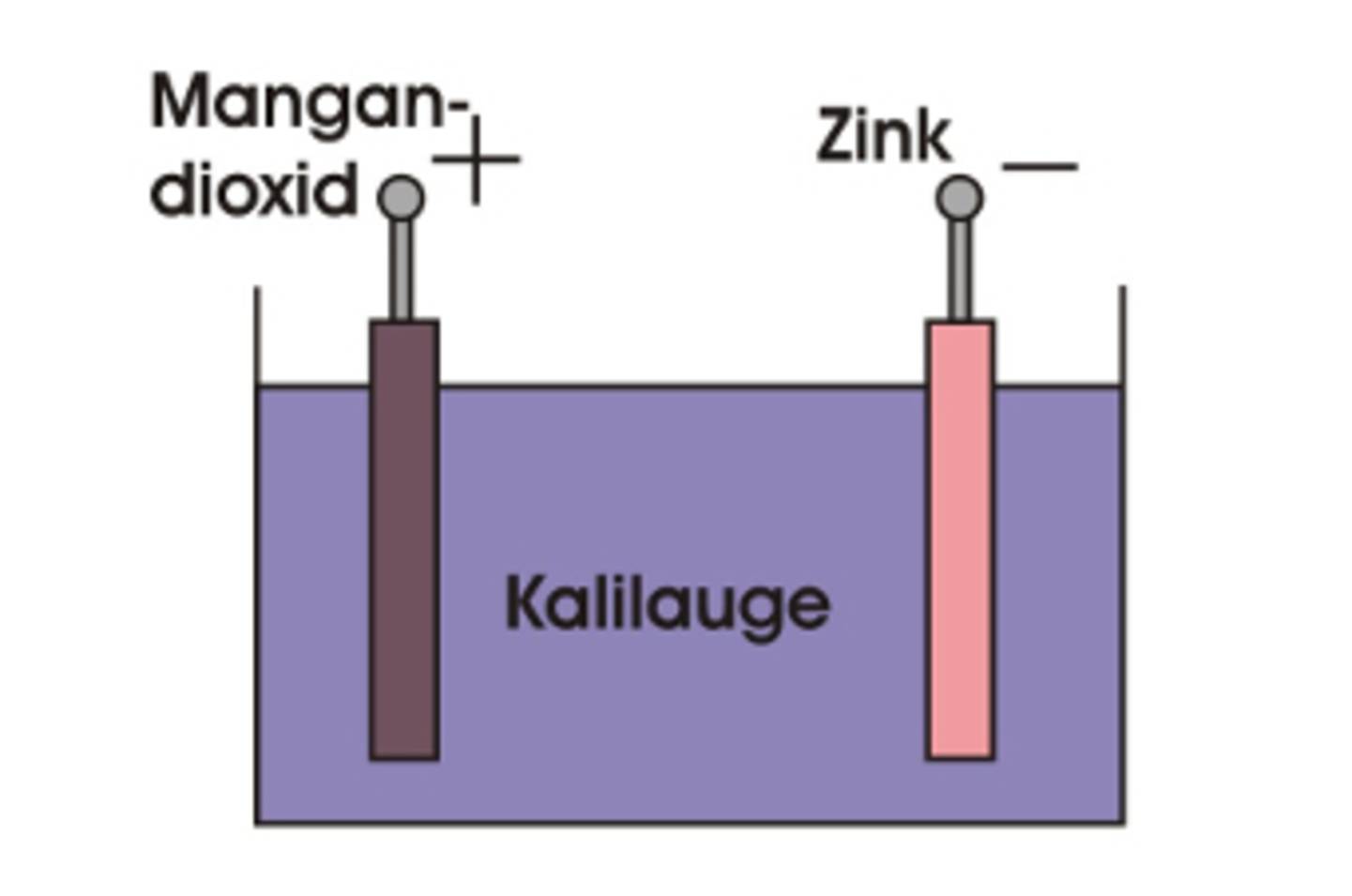
A battery is an energy storage device in which the stored chemical energy is converted into electrical energy by reducing oxidation. In principle, these are two electrochemical substances (electrodes) with different electrochemical voltage potentials, which are housed in a housing with electrolyte (see figure 1).
In the case of an alkaline battery, the two electrodes consist of zinc (minus pole/anode) and manganese dioxide (plus pole/cathode). Concentrated caustic potash (potassium hydroxide) is used as electrolyte, with which the two electrodes and the separator are soaked (see figure 2).
Since batteries are intended for repeated use, they are also called primary cells. In contrast, rechargeable batteries are also referred to as secondary cells.
In colloquial language, however, the terms are often used incorrectly. Even experts speak of a car battery or starter battery, even though these energy donors are rechargeable lead-acid batteries.
Figure 1: Simplified construction of an alkaline battery
Battery or rechargeable battery, what do I use when?

Battery-powered devices often ask themselves whether the use of rechargeable batteries would not be more sensible. Unfortunately, the question cannot be answered in a flat-rate way.
Since rechargeable batteries are subject to a certain self-discharge (up to 60% per month), it can be said, however, that for consumers with a short on-time but with a long on-time, batteries make sense. Classic examples of these consumers would be emergency torches, clocks, TV remote controls or even wireless sensors for weather stations or smart home systems.
If the switch-on times of the consumers are longer, repeat themselves more often or the power requirement is not insignificant, then the rechargeable batteries are the better choice. Radios in construction sites, flashlights for security services or the wireless mouse on the daily office computer should therefore be equipped with rechargeable batteries.
But often every user has to decide for himself whether he prefers to use batteries or rechargeable batteries. In a model construction control, which is only used once in a while, batteries make sense. However, if the model is used regularly, it is more economical and also more sustainable to use rechargeable batteries in the remote control.
Figure 2: High-quality alkaline-manganese batteries are included in an emergency torch
How is a battery structured?
Figure 3 shows the schematic setup of an alkaline manganese battery.
- Metal cup with positive pole on the top
- Outer film with manufacturer labeling
- Manganese dioxide (cathode)
- Plastic sealing washer
- Base plate (minus pole)
- Separator for separating the electrodes and as an ion bridge
- Discharge nail
- Zinc powder gel (anode)
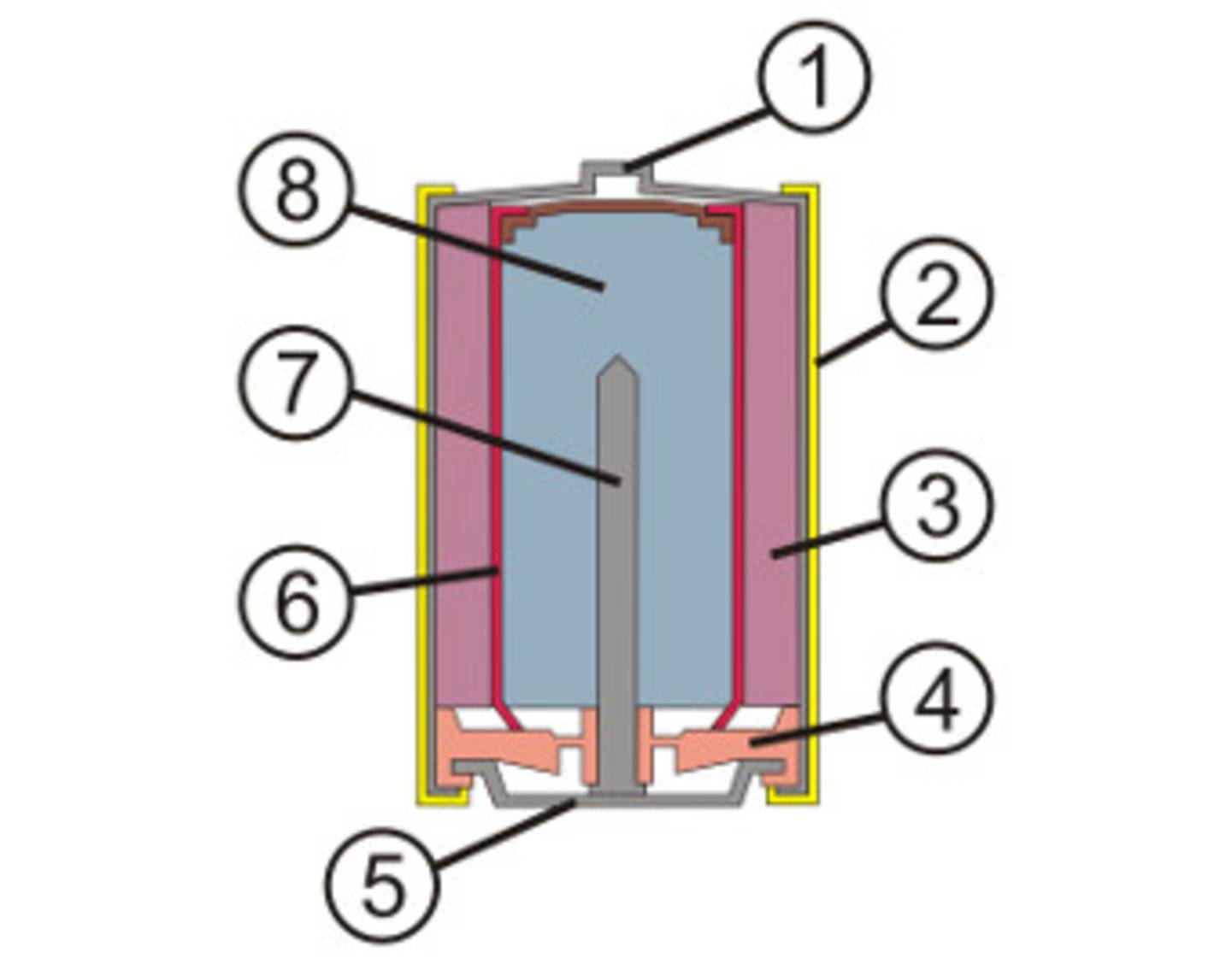
Figure 3: Cut-out drawing of an alkaline manganese battery
Note:
On a zinc-carbon battery, the outer cup was zinc. As the zinc electrode decomposes and becomes pierced during discharging, these batteries often leak when used up.
In an alkaline battery, the outer cup is made of metal and the zinc electrode is located in the Kern of the battery. This reliably prevents battery leakage.
Different chemistry results in different voltages
Since the different substances from which battery electrodes can be manufactured have different chemical voltage potentials, different nominal voltages are also produced for the respective battery types.
- 1.35 V for the mercury oxide zinc cell
- 1.5 V for the alkaline manganese cell
- 1.5 V for the zinc-carbon cell
- 1.4 V for the zinc-air cell
- 1.5 V for the lithium iron sulphide cell
- 1.55 V for the silver oxide zinc cell
- 2.9 to 3.7 V for lithium cells, depending on the cathode material
In the case of a 4.5 V flat battery or 9 V block battery, several individual cells are connected in series within the battery in order to maintain the higher voltage level.
For a 4.5 V flat battery, these are four cells (3 x 1.5 V = 4.5 V) and for a 9 V block battery, these are six cells (6 x 1.5 V = 9 V).
How does a battery work?
In simple terms, an alkaline battery provides the electrical energy by oxidation of the zinc or by reducing the manganese dioxide. Since oxidation and reduction take place simultaneously, it is called a redox reaction. The electrons released are available at the negative terminal of the battery.
If a consumer is operated with the battery, the electrons move from the negative pole (anode) via the consumer, e.g. a torch bulb, to the positive pole (cathode). For charge balancing, hydroxide ions migrate from the cathode to the anode within the battery. The chemical processes that occur in the battery are explained in a very clear way in the video attached below.
How does a battery work?
Battery test without aids
Practical advice
As water is consumed when an alkaline battery is discharged, the battery "dries" internally. For AA batteries, a simple test can be used to check whether the battery is full or empty. The video above shows how to do it.
When the battery is full, the electrolyte is gel-like and thus attenuates the impact on the table top. Once the battery is discharged, the electrolyte is solid and the battery springs on the tabletop several times. Unfortunately, this trick only works with batteries of the size Mignon (AA).
Battery types compared
As already mentioned, battery electrodes can be made from a wide variety of chemical substances. In addition to the resulting different voltages, there are also type-specific advantages and disadvantages, as well as preferred areas of application.
Zinc-carbon
The zinc-carbon batteries (ZnC) are particularly suitable for less demanding applications such as remote controls or wall clocks. These batteries are hardly offered or replaced by cheaper alkalis.
Pros:
- Cheap
Disadvantages:
- not leak-proof
- no high current load
Lithium
Lithium (LiMnO2) batteries have a long life and a very constant cell voltage. Their application is everywhere, where one has to rely on battery current.
Lithium batteries should be used primarily in devices with increased power requirements, such as cameras, digicams, camcorders, laptops, but also in smoke detectors and outdoor sensors. Lithium batteries should also be used in any emergency torch.
Advantages:
- extremely high capacity
- ideal for high-current consumers
- low self-discharge (long storage times)
- wide temperature range (-40 to 60° C)
Disadvantages:
- more expensive than alkaline batteries
Alkali-Manganese
The alkaline manganese (AlMn) or usually only alkaline batteries have a high performance and are long-lasting. The most common application area is in radios, remote controls, toys and watches.
Advantages:
- higher capacity than zinc-carbon
- higher current carrying capacity
- leak-proof
Disadvantages:
- more expensive than zinc-carbon batteries
- temperature sensitive
Battery sizes in comparison
When selecting the right battery, the battery size or design is probably the most important decision criterion in addition to cell chemistry and cell voltage. Finally, the replacement battery must also fit into the battery compartment of the device. Various size designations and standards have been established.
Standard batteries
| General description | ANSI standard | Nominal voltage | Dimensions in mm: | Labeling |
|---|---|---|---|---|
| Lady | N | 1.5 V | Ø x H 12 x 30 | LR1, R1, A1, UM5 |
| Micro | AAA | 1.5 V | Ø x H 10.5 x 44.5 | LR03, R03. AM4, UM4 |
| Mignon | AA | 1.5 V | Ø x H 14.5 x 50.5 | LR6, R6, AM3, UM3, L91 |
| Baby). | C | 1.5 V | Ø x H 26.2 x 50 | LR14, R14, AM2 |
| D cell | D | 1.5 V | Ø x H 34.2 x 61.5 | LR20, R20, AM1 |
| 9V block | 1604D(PP3) | 9 V | L x W x H 26.5 x 17.5 x 48.5 | 6LR61, 6F22, 6AM6 |
| 4.5 V flat battery | - | 4.5 V | L x W x H 67 x 62 x 22 | 3LR12, 3R12, 1203 |
Special batteries;
| General description | ANSI standard | Nominal voltage | Dimensions in mm: | Labeling |
| Mini | AAAA | 1.5 V | Ø x H 8.3 x 42.5 | LR8, LR8D425, LR61, E96 |
| Rod battery | - | 3 V; | Ø x H 21.8 x 74.6 | 2R10, 2R10R, 3010, 2010 |
| Flat Pack | J | 6 V | L x W x H 47 x 34 x 8 | 4LR61, 4018, 7K67, 866, KJ |
| Torch battery | 908D | 6 V | L x W x H 115 x 67 x 67 | 4R25, 4R25C, 430, GP908X |
| A23 battery | V23GA | 12 V | Ø x H 10 x 28 | E23A, V23A, L1028, MN21, ... |
Special battery comparison table
Especially with special batteries, there are a wide variety of designations which lead to confusion over and over again. For this reason, we have summarized the different battery designations in a table for you.
Special alkaline/zinc batteries
| Battery type | Names |
| 10A | A10, E10A, V10A, V10PX, V10GA, L1021, L1022, MN10, G10A, GP10A, WE10A, UM10A, LR10A, K10A, 10AE, P10GA, PX10, EPX10, KX10, RPX10, R10A |
| 11A | A11, E11A, V11A, V11PX, V11GA, L1016, MN11, G11A, GP11A, WE11A, CA21, CX21A, UM11A, LR11A, K11A, 11AE, P11GA, PX11, EPX11, KX11, RPX11, R11A |
| 23A | A23, E23A, V23A, V23PX, V23GA, L1028, MN21, G23A, GP23A, WE23A, CA20, UM23A, LR23A, LRV08, RVO8, MS21, K23A, 8LR932, 8LR23, 3LR50, 23AE, A23S, P23GA, VR22, 8F10R, MN23, PX23, EPX23, KX23, RPX23, 4NR23, R23A |
| 27A | A27, E27A, V27A, V27PX, V27GA, L728, L828, MN27, G27A, GP27A, WE27A, CA22, UM27A, LR27A, K27A, 27AE, A27S, P27GA, EPX27, KX27, RPX27, HS3, NR43, EL812, EL8212, R27A |
| 476A | A476, E476A, V4034PX, V476A, V476GA, L1325, V34PX, GP476A, WE476A, UM476A, LR476A, K476A, 476AE, A476S, P476GA, EPX476, KX476, RPX476, 4LR44, 7H34, 537, 4LR44P, 1414A, K28A, 4L1325, 4G13, R476A |
| 544A | A544, E544A, V28PX, V28PXL, V28GA, V544A, L544, KS28, PX28A, WE544A, PX544A, GP544A, LR544A, K544A, 544AE, A544S, P544GA, KX544, RPX544, 4SR44, 4NZ13, G13, 4028, K544A, R544A, 28L |
| Mini | AAAA, LR8, LR8D425, R8D425, LR61, E96, MX2500, V4004, V4761, MN2500, 25A |
| V74PX | MN154, 504, KA74, 220, 220A, 4074, 10LR54 |
| 6V flat pack | 4LR61, Flat Pack, 4018, 7K67, 866, 539, 1412AP, KJ, J |
| 4R25 | 4R25C, 430, GP908X |
| 4R25-2 | 4R25C, 430, GP908X |
| 4LR25 | MN908, PC915, 4R25-2P, 529, 908A, DC908, 4LR25Y |
| 4LR25-2 | MN918, PC918, 4LR25-24, 4R25-2C, EV31, R25-2, 731, 991, 1231, LR825 |
| 2R10 | 2R10R, 3010, 2010 |
| 6F100 | V439, 439, PP9 |
| U23PX | V23PX, EPX23, PX23, 4SR42, PX23S, RPX23A, 4NR42, 4LR42, RPX23S, PX23A, KX23, RPX23, RX23 |
| U21PX | V21PX, EPX21, PX21, 3LR50, PX21S, RPX21A, RPX21S, PX21A, KX21, RPX21, UG-523, 3MR50, RX21, E523, BK-1, PC133A, 523 |
| TR164 | PX164A, S4164, EPX164A, E164, V164P, PX164, A32PX, HM-4N |
| U72PX | UG015, S4072, MN122, 15LR43, NM412, UG 015, 15F20, PX72, 412, 215, V72, V72PX, A72PX |
| PX27A | PX27, 4AG12, EPX27, 4AG13, S27PX, 4LR43, PX27S, 4SR43, 4NR43. U27PX, HS3C, RPX27S, RPX27A, RPX27, V27PX, KX27, HS-3 |
| LL4 | PS-LL4 |
| CR435 | BR435, 435 pin-type, 435PT |
Special lithium batteries
| Battery type | Names |
| 2CR5 | EL2CR5, KL2CR5, EL2CR5BP, RL2CR5, DL245, DL345, 2CR5M, 5032LC, 245 |
| CR2 | EL1CR2, KCR2, RLCR2, DLCR2, DLCR2B, DR2R, RLCR2-L, 5046LC, CR17355 |
| CRV3 | LB01, CRV3P, RB104358 |
| CR-123A | EL123AP, K123LA, RL123A, EL123A, DL123A, 5018LC, LR123, VL123, CR17345 |
| CR-P2 | EL223AP, K223LA, RLP2, EL223APBP, DL223A, 5024LC, VL223, CR223A, CRP2, CRP2P, CR17-33, CRP2S, PC223A, DL223, K223, PC223, 223 |