AGM batteries » Powerful lead-acid batteries with clever AGM technology
Published: 10.10.2023 | Reading Time: 7 minutes
This text is machine translated.
Long before the boom in electromobility, every driver had a starter battery installed in their vehicle. This was a lead-acid battery that was charged by the alternator while driving.
Lead-acid batteries were inexpensive and could provide the required currents for the electric starter when needed. However, the fact that they were literally heavy as lead did not play such a major role in vehicle batteries. In principle, the starter batteries had a reasonable price-performance ratio and worked so well that no significant further development took place over the years.
The only real problem with the open batteries was the evaporation of water. In order not to shorten their service life, the batteries had to be topped up with a little distilled water from time to time. This only changed when the first batteries with AGM technology were installed in new vehicles.
Two different methods were used to make lead batteries maintenance-free and therefore more user-friendly. Firstly, the liquid diluted sulphuric acid was thickened with silicon dioxide and introduced into the battery as a gel.
These lead-gel batteries could then be hermetically sealed and could therefore be used in any position. However, care must be taken when charging to ensure that the charging voltage is not too high and the battery begins to gas. In an emergency, there is therefore a safety valve for each cell that triggers if the internal pressure is too high.
Another solution was fleece technology, in which the diluted sulphuric acid is still liquid, but is absorbed by a special fleece. The name AGM battery (Absorbent Glass Mat) is also derived from this technology.
Our product recommendations for AGM batteries
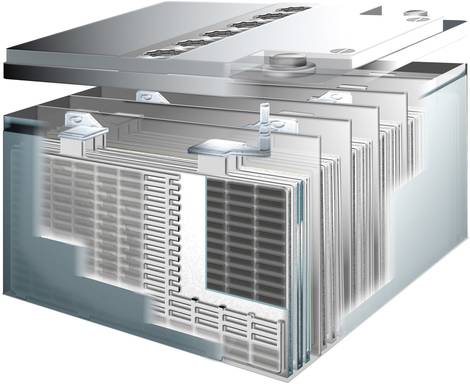
An Absorbent Glass Mat battery is basically a closed lead-acid battery in which the fleece between the lead plates holds the electrolyte, i.e. the diluted sulphuric acid, like a sponge.
Basic structure of lead-acid batteries
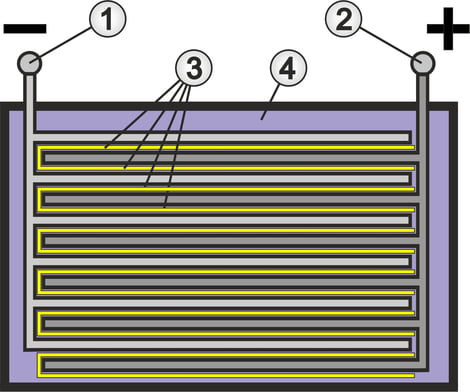
There are two lead electrodes in an acid-resistant container, which are constructed as a grid on the inside (see illustration in the upper section).
The negative electrode (1) is coated with porous lead (Pb), also known as lead sponge. The positive electrode (2) is coated with porous lead dioxide (PbO2). The coatings are also referred to as active mass, as they actively participate in the chemical reaction. The porous structure is necessary to maximize the surface area for the chemical reaction during charging and discharging.
For greater efficiency in terms of capacity and current output, the electrodes are constructed as interlocking groups. The positive plates as well as the negative plates are electrically connected to each other.
There is a corrugated PVC separator (3) between the interlocking plates of each group, which prevents direct contact (short circuit) between the plates. In an AGM battery, the glass fleece acts as a separator.
The electrolyte (4) is sulphuric acid (H2SO4) diluted with water, which enables a charge exchange between the plates. The ratio between water and sulphuric acid is selected in such a way that ideal dissociation, i.e. the decomposition of chemical compounds into molecules, atoms or ions, is achieved. This achieves the optimum ionic conductivity in the electrolyte. Pure sulphuric acid or just water would not work in this case.
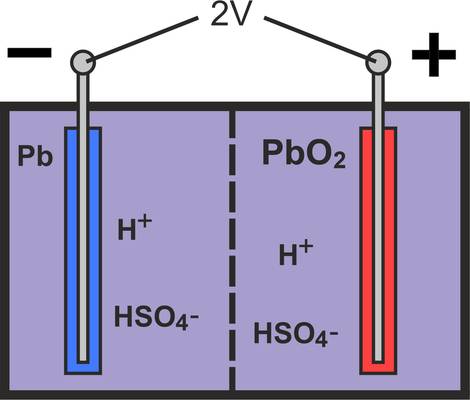
If the container with the electrodes listed above is filled with diluted sulphuric acid (1.28 kg/l), there is an excess of electrons at the negative electrode of the battery and a lack of electrons at the positive electrode.
The potential difference between the two electrodes is around 2 V and can vary between 1.75 and 2.4 V depending on the state of charge. The reason for this becomes clear if you take a closer look at the chemical processes involved in charging and discharging. For a better overview, we have only shown a compact electrode in the drawings in this section.
As a battery with a nominal voltage of only 2 V is unsuitable for vehicle operation, 6 cells are connected in series in a vehicle battery. As a result, the battery achieves a terminal voltage of 12 V (6 x 2 V). For operation in a truck, two 12 V batteries are connected in series so that the vehicle can be supplied with 24 V. This means that the electrical consumers can be supplied without any problems, even when the vehicle is parked for long periods, without starting problems occurring afterwards.
Unloading process
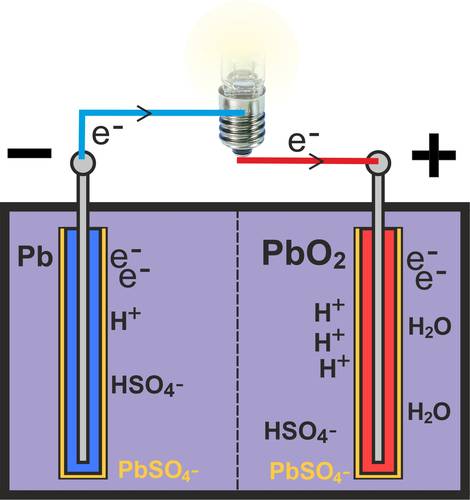
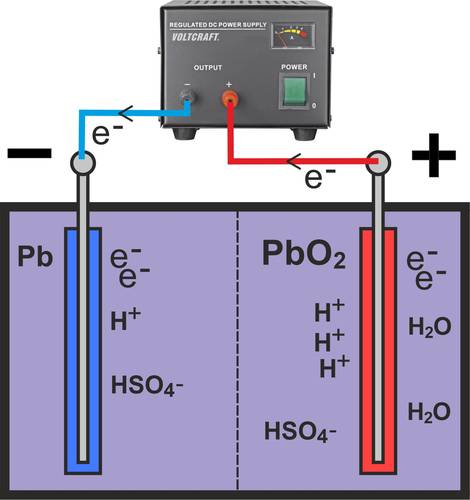
When discharging a fully charged lead-acid battery cell, the cell is discharged via the external circuit (see lamp in picture). Electrons (e-) flow via the external circuit from the negative electrode (negative terminal of the battery) to the positive electrode (positive terminal).
As the sulphuric acid (H2SO4) is present in a dissociated state, the hydrogen sulphate (HSO4-) oxidizes at the negative electrode (Pb) to form porous lead sulphate (PbSO4), releasing hydrogen (H+). At the same time, free electrons (e-) are produced at the negative electrode, which flow to the positive terminal via the external circuit.
The chemical equation is then as follows:
Pb + HSO4- → PbSO4 + H+ + 2e-
A reduction takes place at the positive pole of the lead battery. By absorbing electrons (e-), the lead dioxide (PbO2) of the electrode is reduced together with the hydrogen sulphate (HSO4-) and the hydrogen (H+) to lead sulphate (PbSO4) and water (H2O).
In this case, the equation is as follows:
PbO2 + HSO4- + 3H+ + 2e- → PbSO4 + 2H2O
Looking at the overall reaction, lead, lead dioxide and sulphuric acid react to form lead sulphate and water.
Pb + PbO2 + 2H2SO4 → 2PbSO4 + 2H2O
As a result, the acid concentration in the electrolyte is reduced during discharge.
Charging process
When the charging voltage is applied, electrons are supplied to the negative electrode and removed from the positive electrode. The chemical reactions that took place during discharging now take place in reverse.
The lead sulphate (PbS04) present is reduced to lead (Pb) and hydrogen sulphate (HSO4-) by absorbing hydrogen and electrons. The chemical equation now looks like this:
PbSO4 + H+ + 2e- → Pb + HSO4-
Oxidation now takes place at the positive pole. By releasing electrons, lead sulphate (PbSO4) and water (H2O) become lead dioxide (PbO2), hydrogen sulphate (HSO4-) and hydrogen (H+) again.
In this case, the equation is now reversed:
PbSO4 + 2H2O → PbO2 + HSO4- + 3H+ + 2e-
As a result, the acid content in the electrolyte increases during the charging process, which can also be seen in the overall equation:
2PbSO4 + 2H2O → Pb + PbO2 + 2H2SO4
This is also the reason why the current state of charge of open lead-acid batteries can be checked using a battery acid siphon.
The main advantage of AGM batteries is the high number of charging cycles. This means that the batteries offer a significantly longer service life than conventional wet batteries. When used in vehicles, the low internal resistance enables very high currents for the starting process, which are also available in winter thanks to the high cold stability.
This is why AGM technology is also used in vehicles with a start-stop function. In addition, the batteries are virtually maintenance-free and can be discharged very deeply if necessary without being damaged. Thanks to the Glass Mat technology, the electrolyte cannot leak, even if the housing has been mechanically damaged.
In contrast to conventional lead-acid batteries, AGM batteries have a lower internal resistance due to their design. This enables them to deliver very high currents. As neither acid stratification nor water loss occurs, the batteries are very durable and have a significantly higher cycle stability.
AGM batteries are therefore ideal as starter batteries for vehicles with start-stop technology or with brake energy recovery.
As AGM batteries are completely sealed, they can also be used in motorhomes. But AGM batteries are also used in alarm systems, solar systems, UPS systems or even in electric vehicles (forklift trucks or scooters). Thanks to their low self-discharge rate, they can also withstand long periods of downtime without any problems.
There can be many reasons for the premature failure of lead-acid batteries. We have briefly summarized the most important reasons for failure:
Water loss
A major reason for the short service life or premature failure of lead-acid batteries was the loss of water through evaporation and thus the dryness of the lead plates. With gel or AGM batteries, evaporation is prevented or minimized by the closed housing.
Acid coating
However, acid stratification can also become a problem with lead-acid batteries. During the discharge process, the acid density in the electrolyte drops, which means that the acid content in the sludge space below the plates is significantly higher than in the upper area. In an AGM battery, the fleece reduces the acid stratification.
Sulphation
Another ageing criterion is sulphation, in which the otherwise porous lead sulphate layer takes on a crystalline structure. As a result, it no longer actively participates in the charging and discharging process. This can happen due to the acid stratification mentioned above or if the batteries have been partially or even completely discharged for a long time.
Erosion
Erosion causes active material to fall off the arrester (grid). This occurs as a result of mechanical stress during charging and discharging processes, gassing or high currents. The consequences of this are significant capacity losses, a shortened service life, higher internal resistance and short circuits within the cells.
Korrosion
Corrosion between the grid and the active mass on the positive electrode is just as problematic. In addition to the acid coating and high temperatures, the electrode potential and the material quality are also responsible for grid corrosion. In some cases, manufacturers add antimony, calcium or silver to improve the mechanical and electrical properties.
Our practical tip: Actively avoid sulphation
If possible, lead batteries should always be kept in a fully charged state. Even during storage. To prevent sulphation, the batteries should be recharged from time to time with a suitable charger if they are only used sporadically in vehicles.
Otherwise, sulphation can lead to battery failure. Some companies therefore offer so-called refreshers or pulser, with which crystalline structures can be avoided or existing structures can be broken up again.
Why are AGM batteries not usually installed in the engine compartment?
As AGM batteries do not tolerate high temperatures, they are not installed in the immediate vicinity of the combustion engine.
Can AGM batteries be charged with any conventional charger?
Unregulated transformer chargers are not suitable. Ideally, the charger should work according to the IUoU characteristic curve. This involves initially working with a continuous charging current (I) until the maximum charging voltage is reached. The charging voltage then remains continuously at the same level, whereby the charging current decreases over time. Once the current falls below the minimum charging current, the system automatically switches to trickle charging.
What maintenance work needs to be carried out on AGM batteries?
The maintenance-free batteries only need to be charged regularly. If this is not done automatically during regular operation, a charger must be connected at regular intervals to prevent excessive discharge. A visual inspection of the connection terminals should be carried out and any dirt removed. No further maintenance work is required.
What does capacity mean for a battery?
The capacity of a battery indicates how much current the battery can deliver over a certain period of time. The battery capacity is therefore specified in ampere hours (Ah). A battery with a battery capacity of 18 Ah, for example, can supply electricity for 10 hours.