LiIon technology » The current standard for high-performance batteries
Published: 10.10.2023 | Reading time: 8 minutes
This text is machine translated.
Not so long ago, telephones still stood in the hallway or living room, had a brocade cover and were permanently connected to the socket by cable. Back then, wireless communication devices were only seen in science fiction films.
However, the step towards today's mobile communication was only possible because the development of battery technology also made huge progress very quickly.
The key term for this is the lithium-ion battery, which enables enormous amounts of electrical energy to be stored in a very small space. This means that our smartphones, tablets and notebooks can run for a long time without a power connection or charger.
We will be happy to explain exactly what is behind this technology and how lithium-ion technology works.
The abbreviation LiIon refers to lithium-ion technology and is derived from the chemical element lithium. As soon as lithium has an electrically charged state, it is referred to as a lithium ion. A LiIon battery is therefore a rechargeable power storage device in which lithium ions play the decisive role in charge exchange.
Alternatively, there are also Li-ion batteries, which are used as button cells in watches, for example, and cannot be recharged.
In contrast to the outdated nickel-cadmium batteries or the still current nickel-metal hydride batteries, which have a cell voltage of 1.2 volts, Li-ion batteries have a significantly higher voltage of 3.3 - 3.8 volts per cell. In addition, the energy density, i.e. the storage capacity of electrical energy, is also significantly higher.
Our product recommendations for LiIon batteries
Like any other rechargeable battery or a conventional battery, the Li-ion battery consists of a positive electrode, a negative electrode and a liquid electrolyte that serves as a transport medium for the lithium ions.
The concrete structure is as follows:
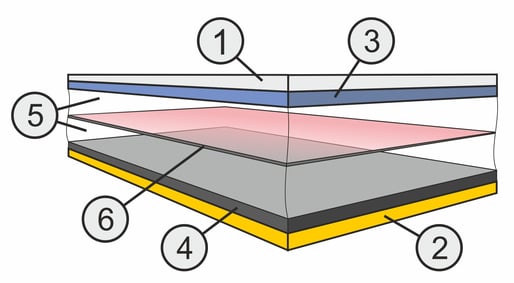
The current is supplied to the electrodes via electrically conductive layers made of different materials. Aluminum (1) is used as the conductive layer for the positive connection and copper (2) is used as the conductive layer for the negative connection.
The positive electrode (+ pole) consists of a lithium metal oxide (3), which can contain different proportions of nickel, cobalt, manganese or aluminum.
Alternatively, lithium iron phosphate is also used as an electrode material.
The negative electrode (- pole) consists of carbon (4) or rather graphite, which is structured in layers.
However, nanocrystalline, amorphous silicon, lithium titanate or tin dioxide are also used instead of graphite.
Important:
The type and composition of the electrodes determine the voltage level and the electrical properties of the battery. However, as a general rule, the more uniform and pure the chemical composition, the higher the performance and the longer the service life of the cell. To enable the lithium ions to move back and forth as charge carriers in the cell, there is an anhydrous electrolyte (5) between the electrons, which serves as a transport medium. Various salts are used, such as lithium hexafluorophosphate or lithium tetrafluoroborate, which are dissolved in aprotic solvents such as ethylene or propylene carbonate. The electrolyte must be extremely pure and also enriched with lithium ions so that the charging and discharging processes can take place undisturbed. In addition, the electrolyte supports the formation of the three-dimensional boundary phases solid electrolyte interphase (SEI) on the negative electrode and cathode electrolyte interphase (CEI) on the positive electrode, which are necessary for the battery to function.
To prevent a short circuit between the electrodes, the electrodes are separated by a separator (6). The separator consists of a microporous polyolefin membrane that is only permeable to the tiny lithium ions.
We will use a common lithium cobalt oxide battery to explain how it works, the internal processes and the chemical reactions during charging and discharging.
The charging process
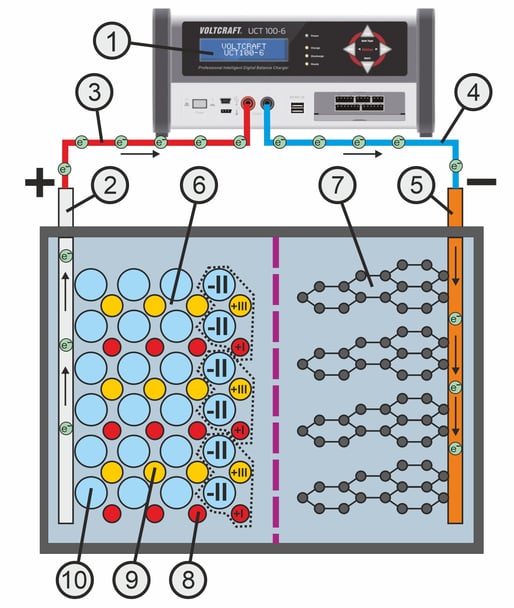
During the charging process, a voltage source or charger (1) is connected to the electrodes.
There is a shortage of electrons at the positive pole of the charger, which means that electrons (negatively charged elementary particles) can be absorbed. There is a surplus of electrons at the negative pole of the charger. Electrons are released here during the charging process.
The charging voltage applied to the battery causes electrons to move from the positive terminal of the battery (2) via the charging cable (3) to the positive terminal of the charger. Similarly, electrons move from the negative terminal of the charger via the charging cable (4) to the negative terminal of the battery (5).
The charging current would cause the lithium metal oxide layer (6) to become positively charged and the graphite layer (7) to become negatively charged.
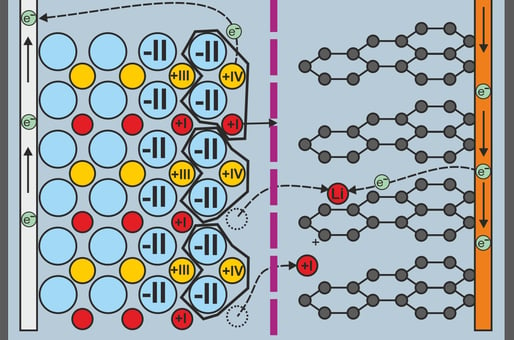
If we now look at the oxidation numbers, i.e. the elementary charges of an atom within a compound, the following picture emerges for the lithium metal oxide layer:
Lithium (8) has the oxidation number +I
Cobalt (9) has the oxidation number +III
Oxygen (10) has the oxidation number -II
The lithium cobalt dioxide (LiCoO2) with its two oxygen components, a cobalt element and a lithium ion, is therefore electrically uncharged in itself.
Processes within the cell during the charging process
The charging process removes electrons at the cathode, which further oxidizes the cobalt to oxidation state IV. The lithium cobalt dioxide would then become positively charged. To counteract this, the lithium ions leave the oxide, making it uncharged again.
The now free lithium ions migrate through the electrolyte and the separator to the positive terminal of the battery. There they form an intercalation bond (Latin intercalare = to insert) with the layered structure of the graphite, absorbing the free electrons present due to the charging voltage.
When all the lithium ions have arrived at the negative electrode and are embedded, the battery is fully charged.
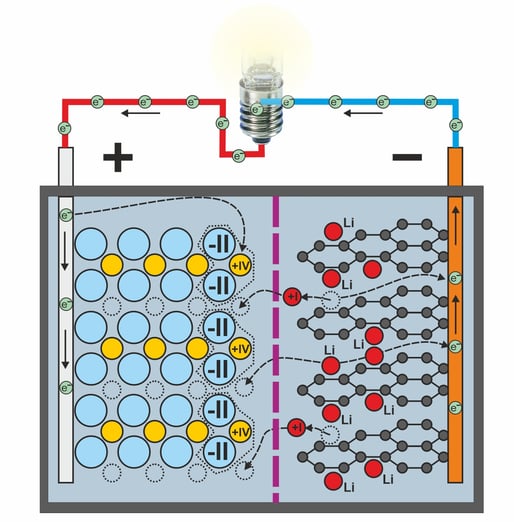
The unloading process
When discharging, the intercalation compounds on the negative electrode are released. The lithium releases the electrons it has absorbed and becomes a positively charged lithium ion.
Since metallic lithium, as a base metal, releases its electrons extremely easily, this process is virtually automatic. All that is required is to connect the electrodes of the battery to an external circuit, including a consumer.
The electrons emitted by the lithium are conducted via the consumer circuit from the negative terminal via the consumer to the positive terminal.
There they reduce the cobalt from oxidation state IV back to state III. This means that the cobalt dioxide is negatively charged and can now absorb positively charged lithium ions again.
And this is exactly what happens. After the electron is released, the lithium ions migrate from the negative electrode through the electrolyte and the separator back to the positive electrode. There they recombine with the cobalt dioxide to form LiCoO2.
The term LiIon battery is more or less a generic term for a whole family of batteries that differ in terms of the chemical composition of the cathode. The most important representatives are
Overview of the most common lithium-ion batteries
| Description | Cathode material | Type | Cell voltage | Energy density | Number of cycles |
|---|---|---|---|---|---|
| Lithium cobalt oxide battery | LiCoO2 | LCO | 3.6 Volt | 200 Wh/kg | 500 - 1000 |
| Lithium-manganese battery | LiMnO2 / LiMnO4 | LMO | 3.7 – 3.8 Volt | 150 Wh/kg | 300 - 700 |
| Lithium-nickel-manganese-cobalt battery | LiNixMnyCozO2 | NMC | 3.6 – 3.7 Volt | 220 Wh/kg | 2000 |
| Lithium-nickel-cobalt-aluminum battery | LiNixCoyAlzO2 | NCA | 3.6 – 3.7 Volt | 250 Wh/kg | 1000 |
| Lithium-iron-phosphate battery | LiFePO4 | LFP | 3.2 – 3.3 V | 170 Wh/kg | > 4000 |
We have listed the different performance features and characteristics, as well as further information on some of the battery variants, on the linked guide pages.
Advantages:
As already mentioned, lithium batteries are capable of delivering a great deal of energy. The voltage per individual cell as well as the capacity are significantly higher than with conventional batteries. But the cells also offer other advantages such as:
- High efficiency
- Low weight
- Considerable power output
- Fast charging possible
- Minimal self-discharge
- High number of charging cycles
- Long service life
- No memory effect
Disadvantages:
Unfortunately, LiIon batteries also have some disadvantages, but these are not so serious if they are handled correctly and carefully during operation.
State of charge and temperature range
The most important thing is the correct charging process or charge level. This should normally be between 30% and 80% in order not to shorten the expected service life unnecessarily. Charging to 100% only makes sense if the battery is discharged immediately afterwards during use. Deep discharging should be avoided at all costs, as this will damage the battery.
Electronic protection circuits are integrated into smaller lithium cells to effectively prevent overcharging or deep discharging.
But temperature also plays an important role. Lithium batteries cannot tolerate extreme cold or extreme heat. For this reason, battery management systems (BMS) are always used for large battery packs in electric vehicles. A BMS monitors and optimizes the state of charge of the battery or, together with ventilation or heating systems, ensures the correct temperature.
Important!
For batteries without any protective devices, such as those used in model making, users must use suitable chargers including balancers and ensure that no deep discharge or overheating occurs during use.
Due to their performance characteristics, LiIon batteries are popular and widely used. We have briefly summarized the most important areas of application for you:
Small appliances
The high energy density enables a very compact design despite the required capacity, which is also reflected in the low weight.
This is why these batteries are ideal for powering small portable devices such as smartphones, notebooks, tablets, cameras or power tools.
LiIon batteries are also the preferred choice for mobile energy storage devices such as power banks.
E-mobility
Low weight and high energy density also play an important role in vehicles such as electric cars, e-bikes and electric scooters.
After all, ever larger battery packs are required in this area of application.
On the one hand, the high current carrying capacity of the cells gives electrically powered vehicles enormous performance potential and, on the other, ensures very short charging times.
Industrial applications
In the context of the energy transition, efficient and affordable storage systems for electricity from renewable energy sources such as wind and solar are becoming increasingly important.
This not only applies to electricity storage for small solar systems, which are becoming increasingly common on private homes.
The various energy suppliers are also increasingly using large-scale storage systems to relieve the main power lines during peak consumption periods.
There are, of course, many other possible applications for Li-ion batteries. Simply using them in place of heavy and less efficient lead batteries, for example for forklift trucks or driverless transport systems, is an extremely wide range of applications, although this is only one of many possible applications for Li-ion technology.
What is the difference between a Li-ion battery and a lithium-ion battery?
In German, the word "Akku" is used for a rechargeable energy storage device, whereas batteries are intended for single use. However, the term "battery" is increasingly moving away from the non-rechargeable primary cell to become a generic term for energy storage devices. As a result, rechargeable batteries with Li-ion technology are increasingly being referred to as lithium-ion batteries or simply lithium batteries.
What are LiPo batteries?
LiPo batteries are based on lithium-ion technology. The term LiPo stands for lithium polymer and refers to the electrolyte. The electrolyte in these batteries is not in liquid form, but is bound in a gel-like polymer plastic. Lithium polymer batteries are manufactured in a wide variety of designs and some do not have a fixed housing. Instead, they are equipped with a special film coating during production. Due to the resulting reduced weight, LiPo batteries are preferably used for operation in electrically powered model aircraft and other weight-relevant systems. In addition to their thermal and electrical sensitivity, foil-covered LiPo batteries are also not particularly resilient mechanically. Multi-cell LiPo battery packs for consumer devices are often equipped with a battery management system (BMS) integrated into the battery pack.
What does the specification 7.4 V 3000mAh 20C on a battery pack mean?
As a voltage of 7.4 V is specified, this is a battery pack with 2 cells of 3.7 V each connected in series. The capacity of the battery is 3000 mAh. This means that the battery can supply a current of 300 mA for 10 hours. At higher currents, the usable capacity value is reduced. The specification 20C refers to the maximum permissible discharge current. The indication "C" refers to the capacity value. In this case, the maximum discharge current is 20 x 3000 mA = 60,000 mA or 60 A. The maximum charging current is often also specified in this way. With a charging current of 2C, the battery may be charged with a maximum of 6 A.
What is battery management?
In addition to monitoring the permissible limit voltages during charging and discharging, the BMS also ensures an even charge distribution within a battery pack. This is important because otherwise the voltages across the individual cells could be different in a series connection. This is particularly important when charging in order to avoid overcharging the lithium-ion cells. For this reason, model batteries are also equipped with special balancer connections, as in this case the charger also functions as a BMS.
What is a lithium titanate battery?
In a lithium titanate battery, the graphite electrode normally used in lithium batteries has been replaced by a lithium titanate electrode. The nanostructure on the surface ensures that the lithium ions can reach the electrode more easily and also prevents the formation of a surface coating that is impenetrable to lithium ions.