NiMH rechargeable batteries » Proven power storage with nickel-metal hydride
Published: 23.10.2023 | Reading time: 7 minutes
This text is machine translated.
Many small electrical appliances for mobile use are equipped with a battery compartment ex works. The absolute classics in this area are flashlights, which are available in a wide variety of designs and sizes. However, cordless keyboards and computer mice, radios and model-making remote control transmitters are also often powered by batteries.
However, as soon as the devices are used more frequently or for long periods of time, batteries are neither economically viable nor ecologically justifiable. In this case, rechargeable batteries are the far better alternative.
For many years, rechargeable batteries with nickel-cadmium technology (NiCd) were the ideal battery replacement. However, the EU has restricted the use of these batteries across the board due to the toxic heavy metal cadmium, meaning that NiCd cells are only permitted for a few applications.
Nickel-metal hydride (NiMH) batteries are now used as a replacement, which are less harmful to the environment and also provide more power. We would be happy to explain NiMH battery technology to you in more detail and reveal some insider information.
The abbreviation NiMH stands for nickel metal hydride and refers to the electrode materials used. The term metal hydride describes a compound of metals and hydrogen. The principle of reversible storage of hydrogen in a special metal alloy was developed back in the 1960s. The nickel-metal hydride batteries based on this have been on the market since 2006. Even though a NiMH cell has a significantly higher energy density and longer service life than a NiCd battery, it does not achieve the performance level of a lithium-ion battery.
However, NiMH batteries have a decisive advantage: they are nowhere near as sensitive to overcharging and deep discharging as a lithium battery, for example. In addition, the NiMH cell voltage of 1.2 volts is almost at the same level as a battery, which has an electrical voltage of 1.5 volts per cell. Even though batteries have a slightly higher voltage, most devices can also be operated with rechargeable NiMH batteries instead of batteries. This is why Ni-MH rechargeable batteries are the perfect battery replacement.
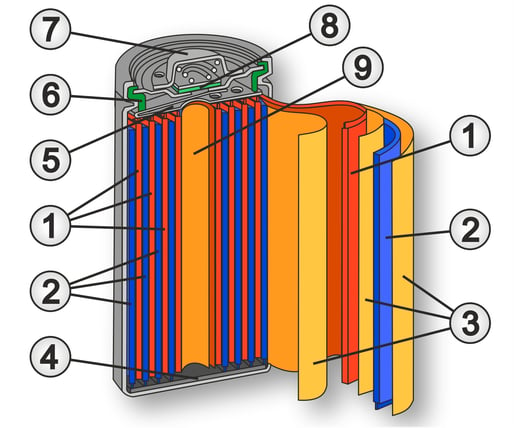
This is also the reason why these rechargeable batteries are mostly offered in the classic battery designs (see also cover picture). However, the batteries are also available in special designs and sometimes with solder tabs for hard-wired connection in electronic circuits.
Our product recommendations for NiMH rechargeable batteries
Our practical tip: Other battery alternatives
At first glance, you might think that the voltage difference between NiMH batteries with 1.2 volts per cell and batteries with 1.5 volts per cell is not that great. After all, the difference is only 0.3 volts. However, if the difference is considered as a percentage, a NiMH rechargeable battery has exactly 20% less cell voltage than a battery. Occasionally, this can lead to problems with devices that are sensitive to an operating voltage that is too low. In this case, nickel-zinc rechargeable batteries, which are rechargeable and have a voltage of 1.6 volts per cell, can help. The rechargeable batteries are available in different sizes, such as Mignon or Micro.
A rechargeable battery with NiMH technology is constructed like most rechargeable batteries. There are two electrodes in a container, which are electrically separated from each other by a separator (e.g. polyolefin). The electrodes themselves consist of a carrier grid to which the active electrode mass is applied.
The negative electrode of a NiMH rechargeable battery consists of a nickel alloy that is able to store hydrogen.
The material composition of the positive electrode depends on the battery's state of charge. When the battery is charged, the positive electrode consists of nickel(III) oxyhydrate. When the battery is discharged, the electrode consists of nickel hydroxide.
The size of the negative electrode determines the capacity of the cell and therefore the amount of electrical energy that can be stored. The negative electrode is designed to be significantly larger than the positive electrode so that there is still enough hydrogen available at the end of the discharge process to be oxidized and the metal is not oxidized.
The electrolyte is a 20% potassium hydroxide solution (KOH) with a pH value of 14.
As with all rechargeable batteries, electrical energy is converted into chemical energy during the charging process in a NiMH rechargeable battery. When discharging, the chemical energy is converted back into electrical energy. To do this, certain processes take place within the cell, which we would like to look at in more detail.
Charging process
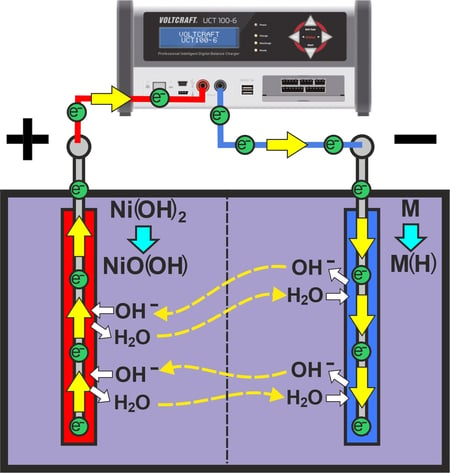
When the charging voltage is applied, negatively charged electrons are removed from the positive terminal of the battery and made available to the negative terminal of the battery.
When discharged, the positive electrode consists of nickel(II) hydroxide. By releasing electrons, the positive electrode oxidizes to nickel(III) oxyhydrate.
To do this, the electrode absorbs hydroxide ions from the electrolyte and releases water into the electrolyte.
At the negative electrode, the water is broken down into hydrogen and hydroxide ions by absorbing electrons, whereby the hydrogen is absorbed by the electrode and stored in the structure.
Positive electrode: Ni(OH)2 + OH- → NiO(OH) + H2O + e-
Negative electrode: M + H2O + e- → M(H) + OH-
Ni(OH)2 = Nickel(II) hydroxide
NiO(OH) = Nickel(III) oxyhydrate
OH- = Hydroxide Ion
H2O = Water
e- = Electron
M = Elemental metal (nickel alloy)
M(H) = Metal enriched with hydrogen
Note:
The water produced by the positive electrode during charging is decomposed again by the negative electrode. In contrast, the hydroxide ions produced at the negative electrode are absorbed by the positive electrode. As a result, there is no change in concentration within the electrolyte during charging.
Discharge process
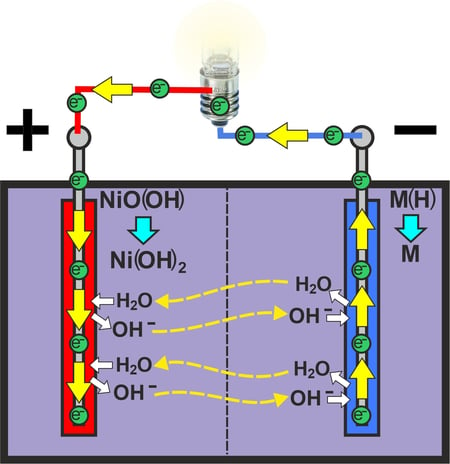
When the two electrodes are connected via an external circuit, the electrons move from the negative terminal via the consumer to the positive terminal.
The chemical processes within the battery are now exactly the opposite of the charging process.
The negative electrode absorbs hydroxide ions and generates water molecules with the hydrogen that is stored in the electrode. The electrons released in the process are released into the circuit.
At the positive electrode, the electrons coming via the circuit reduce the nickel(III) oxyhydrate back to nickel(II) hydroxide. To do this, water is absorbed from the electrolyte and hydroxide ions are released into the electrolyte.
Negative electrode: M(H) + OH- → M + H2O + e-
Positive electrode: NiO(OH) + H2O + e- → Ni(OH)2 + OH-
Note:
The water produced by the negative electrode during discharge is decomposed again by the positive electrode. In contrast, the hydroxide ions produced at the positive electrode are absorbed by the negative electrode. Therefore, there is no change in concentration within the electrolyte when discharging.
As already mentioned, rechargeable NiMH batteries are an ideal environmentally friendly replacement for batteries. However, not every mobile device that runs on batteries is also suitable for rechargeable batteries. The issue here is not the slightly lower cell voltage of the rechargeable batteries. Rather, it is about the power requirement and the frequency of use.
Here are a few examples:
The remote control for a Smart TV, for example, is usually powered by AA or micro batteries. If high-quality batteries are used here, they will work for several months as the power consumption is extremely low. A conventional NiMH rechargeable battery (without LSD technology) would have to be removed and recharged several times during this period, as the self-discharge rate of NiMH batteries is many times higher than the actual power consumption of the remote control.
An emergency flashlight, which is ready to hand in the event of a power failure and illuminates the way to the fuse box in the dark, should contain high-quality batteries. The flashlights of a fuse service, which must work reliably for several hours every night, can be operated with NiMH rechargeable batteries.
Correct battery care
Every battery is only as good as its care. This is especially true for NiMH batteries. Proper battery care begins immediately after purchase. In order for a NiMH battery to develop its full capacity, it must first be charged and discharged several times. Experts refer to this as forming the battery.
High-quality chargers have a separate charging program setting for forming and can do this automatically. In contrast to lead batteries or lithium batteries, which do not tolerate being deeply discharged, NiMh batteries should only be charged when they are empty. This is why many chargers offer the "discharge/charge" function, so that even partially discharged batteries can be fully charged again.
Another important point is self-discharge. This is greatest for batteries with a higher capacity and can be around 5 to 10% on the first day before leveling off at room temperature to around 0.5% per day.
For this reason, NiMH batteries should preferably be charged immediately before use.
Manufacturers are also aware of this problem, which is why LSD (Low Self Discharge) NiMh batteries have been developed, which have a significantly lower self-discharge rate.
Depending on the manufacturer, these batteries are often offered with the addition "Ready to use" or "Always Ready".
Our practical tip: NiMH batteries in cordless phones
Cordless DECT telephones are also often fitted with NiMH batteries. If the handsets spend the whole day in the charging cradle and are recharged immediately after every call, the batteries also suffer. It is better not to place the phone in the charging cradle until the battery is empty or almost empty. For this purpose, the current charge status of the battery can be read off the display on most phones.
What is the memory effect?
The memory effect occurs when a battery is constantly only discharged to a small extent and then immediately recharged. If this battery is then completely discharged, it can only absorb a very small part of the possible charge capacity when charging. However, this effect only occurs with rechargeable batteries with NiCd technology. There is a similar phenomenon with NiMH batteries, which is known as the battery inertia effect or lazy battery effect. However, the effect is not as pronounced with NiMH as with NiCd and can be easily eliminated by repeated charging and discharging.
Can NiMH batteries also be charged with NiCd chargers?
It is not advisable to use old chargers that are only designed for NiCd batteries for nickel-metal hydride batteries. Especially if the chargers are capable of fast charging and provide high charging currents. This is because the voltage drop at the end of the charging curve (-∆U), which is used to detect a fully charged battery, is not as high or as pronounced with NiMH technology. It is better to use a special charger that is explicitly designed for NiMH batteries.
What does the 2000 mAh label mean?
The mAh stands for milliampere per hour and indicates the capacity of the battery. In this case, a battery with 2000 mAh would be able to deliver a current of 200 mA for 10 hours.
Why does the battery not reach the printed capacity?
Chargers with a capacity indicator can display the charged or discharged capacity relatively accurately. However, the capacity information given in the technical data sheets or printed on the battery always refers to values that have been measured in the laboratory and under optimum conditions. The technical data is therefore correspondingly high. The discharge time is around 10 hours, whereby the discharge current is adapted to the discharge time. With higher currents and the associated shorter charging and discharging times, the usable capacity of the battery also decreases.
What characterizes the quality of a rechargeable battery?
Rechargeable batteries are available in a wide range of price categories. High capacity is not always the decisive factor. This is often achieved at the expense of internal resistance. High-quality rechargeable batteries have good capacity values and low internal resistance, which means that the voltage of the battery does not drop or collapse even at higher currents.