NCA Battery & Rechargeable Battery » The Nickel-Cobalt-Aluminum Technology
Published: 10.10.2023 | Reading Time: 3 minutes
This text is machine translated.
In addition to LFP technology or NMC technology, rechargeable batteries with NCA technology represent another important group in the large family of lithium rechargeable batteries. The abbreviation NCA stands for nickel, cobaltand aluminumand describes the composition or the chemical compounds of the positive electrode of the battery.
As a reduction takes place at the positive electrode during discharge, experts also refer to it as a cathode. Consequently, lithium-nickel-cobalt-aluminum oxides are used as the cathode material in an NCA battery.
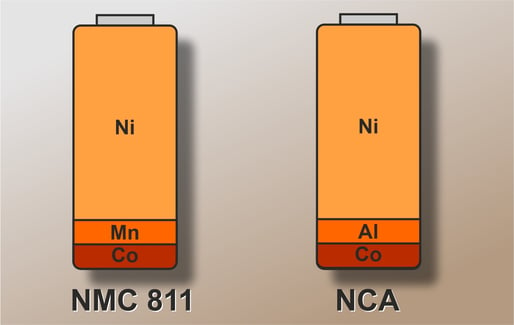
Also worth noting: NCA batteries are very closely related to NMC 811 batteries. They have the same layer structure of the cathode material and also a very similar electrochemical behavior.
For this reason, NMC batteries and NCA batteries are often grouped together when it comes to the safety of the chemical composition or comparison with other lithium battery types such as LFP batteries.
The structure of an NCA battery and its technology corresponds to that of a conventional lithium-ion battery. The percentages of the respective cathode components are very similar to an NMC 811 battery. The only difference is that aluminum is used instead of manganese.
The use of aluminum can increase cycle stability and safety . However, the aluminum ions do not participate in the reduction or oxidation process, which reduces the usable capacity.
As with NMC batteries, developers are trying to reduce the high costs of NCA batteries as far as possible by using the comparatively expensive cobalt only in the quantities that are absolutely necessary.
This is why the nickel-cobalt-aluminum oxides of a nickel-rich NCA battery consist of around 80% nickel. In addition to saving costs, nickel also helps to increase the voltage level and thus increase the amount of energy that can be stored.
We have described the basic function of a lithium battery and the charging and discharging processes in detail in our guide to lithium batteries.
Like all rechargeable batteries that work with lithium-ion technology, NCA rechargeable batteries have both advantages and disadvantages.
Advantages
Compared to NMC batteries, batteries with NCA chemistry have a slightly higher energy density and even better performance potential. In addition, batteries with NCA cathodes have very good fast-charging capabilities. This makes them virtually predestined for use in electromobility.
Disadvantages
As with NMC 811 batteries, the nickel content of NCA cells is very high at approx. 80%. Thermal stability suffers as a result. Thermal runaway or sudden spontaneous combustion, which normally only occurs at high temperatures (approx. 180 °C), can occur at 65 °C if overcharged.
Due to the aforementioned high performance, batteries with nickel-cobalt-aluminum oxide are very popular in the automotive industry.
The US manufacturer Tesla in particular uses drive batteries with NCA technology in its vehicles alongside NMC and LFP cells.
The fact that the cycle stability (see following section) of NCA batteries is not quite as high as that of NMC batteries, for example, is not so important.
Rather, the high energy density is the reason why the cell is currently relatively popular.
Manufacturers of rechargeable batteries and electric vehicles do not always specify the possible charging cycles for their products. This is partly for good reason. After all, if exaggerated fantasy values were not used, one could quickly become puzzled when looking at the real figures. In contrast to rechargeable batteries with NMC technology, for which the manufacturers state a number of cycles of around 2000, rechargeable batteries with NCA technology only manage 1000 cycles. At first glance, this figure does not seem very high.
However, if we assume that an electric car with a full battery can drive a distance of around 300 km, that would be an impressive 300,000 km with 1000 cycles. A value that many vehicles with a combustion engine cannot achieve.
However, the battery is not completely discharged on every journey . On short trips, often only a small percentage of the capacity is used. If the battery is then re charged, this is not a complete cycle.
In order to maintain the performance of an electric car battery in the long term and not accelerate the ageing process unnecessarily, it is essential to follow the advice on battery care that every vehicle manufacturer provides to its customers.
Practical tip: Cycle stability of starter batteries
A classic car battery in a combustion engine vehicle manages an average of around 120 cycles when fully discharged. Although high currents are required for the main task, i.e. supplying power to the starter motor when the engine is started, these only account for a fraction of the battery's capacity value due to the short duration. When the engine is running, the car battery is immediately fully recharged. As the starting process is not a cycle in this case, several thousand starting processes can be successfully carried out within a longer period of time with an intact battery.